
He argued that the cathode ray consisted of small charged particles. Single territory for trade global for academic. Answer (1 of 7): Born on 18 the December1856 and died on 30th August 1940. Thomson had found a charged particle that had a specific. It is about one meter in length and was made entirely by hand. The image below of a CRT used by Thomson in his experiments.
Only the end of the CRT can be seen to the right-hand side of the picture.

Print and/or digital / eBook, including for use in on-line academic databases. Thomson and a cathode ray tube from around 1897, the year he announced the discovery of the electron. Image for Magazines and Journals Book use In the experiment, he had empty glass tubes which he used to. Image for Website or Social Media Magazines and Journals The Thomson Cathode Ray Tube experiment was done in the nineteenth century by J. Web display, social media, apps or blogs. Image for Presentation Website or Social Media Non commercial or internal within a company or organization. When 40kV is applied across the tube, the lower vanes directly between the cathode and anode pivot away from the cathode, causing the wheel to move.
#Jj thomson cathode ray tube free
Not for commercial use, not for public display, not for resale. A paddle wheel is suspended by its axle inside a Crookes tube so that when the paddle vanes spin the entire wheel is free to travel the length of the tube. Thomson died on Augin Cambridge, United Kingdom.Personal Prints, Cards, Gifts, Reference. Thomson’s experiments was clearly stated in the introduction to his 1897 paper. His 1897 experiment on cathode rays is generally regarded as the discovery of the electron. Sir Joseph John Thomson was awarded the Nobel Prize in Physics in 1906. In this section I will discuss the grounds for belief in the existence of the electron by examining J.J. He invoiced a powerful tool in analytical chemistry known as the mass spectrometer. This is one of the original vacuum tubes used by the Cambridge professor of physics Joseph John Thomson (1856-1940) to.

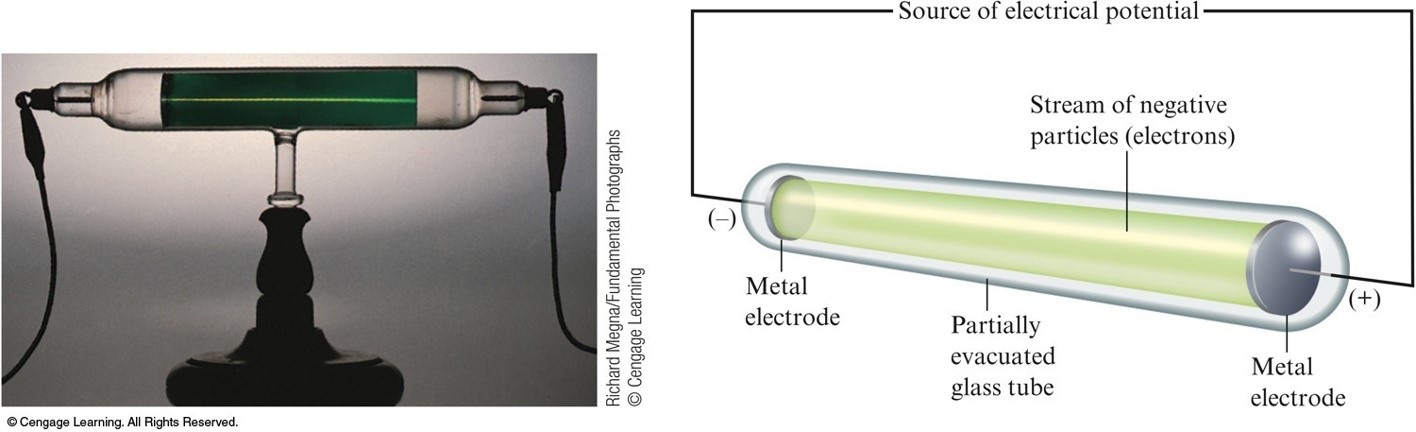
Following this, JJ Thomson also found the first evidence that stable element can exist as isotopes. This model was successful in the fact that it did explain some of the electrical properties of the atom due to the electrons, but it did fail to recognize the positive charges in the atom as particles. Having the desire to be a visualist, JJ Thomson later proposed the plum pudding model for the structure of the atom which included the embedment of negatively charged electrons into a positively charged "soup." His visual showed raisins as the negative electrons and the dough contained the positive charge. It was concentrated, but not localized, at the end of the tube.12. He constructed a partially evacuated glass tube called cathode ray tube. was the mass of ether bound up by the vortex tube. In addition, Thomson studied positively charged particles in neon gas. Cathode rays In the late 1800s an English physicist named J.J.Thomson. He demonstrated that cathode rays were negatively charged.

Thomson's electron discovery was the result of experimenting with a Crookes, or cathode ray, tube. Thomson was a British physicist who helped revolutionize the knowledge of atomic structure by his great discovery of the electron, the first subatomic particle discovery, in 1897. Sir Joseph John Thomson, commonly referred to as JJ Thomson, was born Decemin Cheetham Hill in Manchester, England.


 0 kommentar(er)
0 kommentar(er)
